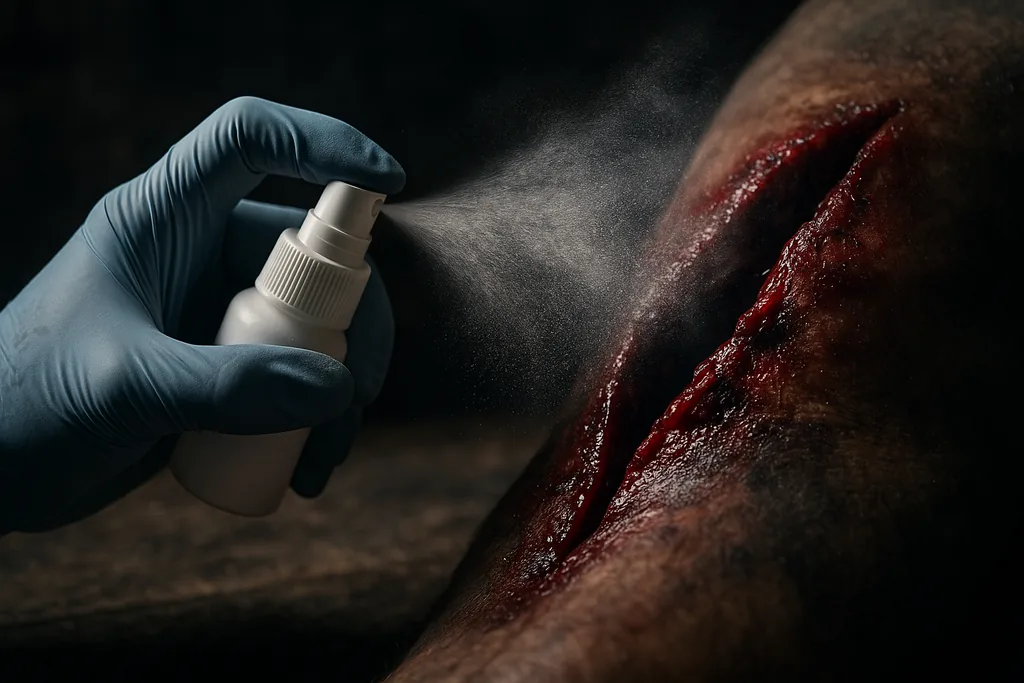
Instant seal: a powder that gels in a heartbeat
This week researchers at the Korea Advanced Institute of Science and Technology (KAIST) unveiled a powder‑based hemostatic agent that, when sprinkled onto a bleeding wound, reacts with blood to form a tough hydrogel barrier in roughly one second. The material — described in a peer‑reviewed article accepted in Advanced Functional Materials and presented in KAIST’s research repository as AGCL powder — is designed to treat deep, irregular or high‑pressure bleeds that are hard to handle with bandages or patch dressings.
Instant chemistry and composite design
AGCL is a deliberately simple mix of biopolymers and a crosslinker: alginate and gellan gum (polysaccharides that gel in the presence of calcium ions), chitosan (a positively charged polymer that adheres to blood and has antimicrobial properties), and a glutaraldehyde crosslinker that helps form a stable network. The powder takes advantage of calcium already present in blood to trigger ionic gelation; when the particles encounter blood, the network snaps shut and a cohesive hydrogel builds up in about one second. The KAIST team reports a very high blood uptake ratio — roughly 725% of the powder’s own weight — which helps the material to quickly expand into a three‑dimensional sealing structure.
Benchmarks: strength, safety and storage
Animal models and healing outcomes
Beyond simple bench tests, the study ran multiple bleeding models — including skin and liver wounds in rodents and larger animal experiments — and compared AGCL against a clinical benchmark dressing (TachoSil). The KAIST team reports significantly reduced blood loss and a shorter time to hemostasis with AGCL. They also observed faster re‑epithelialization, increased angiogenesis (new blood‑vessel growth) and greater collagen deposition in healing tissue, metrics consistent with higher‑quality wound repair. In a liver surgery model the researchers noted that liver function returned to normal within two weeks after treatment with AGCL.
From the lab to the battlefield: design choices
Where this sits among other rapid hemostats
Rapid hemostasis is an old but still active engineering challenge. Commercial products include fibrin and thrombin dressings, collagen sponges and patch‑type sealants; some newer approaches have used nano‑coatings or thrombin‑laden sponges to accelerate clotting. What sets AGCL apart is the combination of ionic gelation for near‑instant physical sealing, high fluid uptake to handle large blood volumes, adhesive strength for high‑pressure leaks, and claimed long ambient storage stability. The KAIST paper used TachoSil as a clinical comparator and reports superior performance in their preclinical models.
Opportunities and limitations
The potential applications are clear: junctional and deep wounds on battlefields, mass‑casualty events, remote trauma care, ambulances and settings without refrigeration or advanced surgical facilities. A sprayable powder that reliably stops life‑threatening hemorrhage in seconds could materially change prehospital survival rates in those circumstances. Several news outlets covering the KAIST announcement emphasised the material’s stability, compact packaging potential, and suitability for harsh environments.
But important caveats remain. The published data are preclinical: safety and efficacy have been demonstrated in vitro and in animal models, not yet in humans. The formulation includes a glutaraldehyde crosslinker, a reactive chemical long used in biomaterials but known in some contexts for cytotoxicity — the KAIST team reports good cytocompatibility in their assays, but regulatory authorities will demand careful toxicology, dosing and clearance studies in people. Clinical usability questions also remain: how to remove or manage the hydrogel during subsequent surgery, whether the powder could obscure surgical fields or cause embolic risk in vascular injuries, and how the product behaves in infected or contaminated wounds. These are normal hurdles on the path from exciting preclinical result to an approved medical product.
Regulatory path and next steps
The KAIST article was accepted in Advanced Functional Materials and appeared online in late 2025; subsequent press coverage has appeared in January 2026. The researchers and their institutional summaries frame AGCL as a strong candidate for next‑generation topical hemostats, but human trials and regulatory review will be required before any frontline deployment. Several outlets that summarised the work explicitly note the technology is not yet approved for clinical use and remains at the research stage. Industry partners, military medical branches or startup spin‑outs typically shepherd materials like this through scaled manufacturing, formal preclinical GLP toxicology and phased human trials before wider fielding.
Ethics, access and use in conflict zones
Deploying a powerful trauma intervention raises both operational and ethical questions. On the positive side, a stable, easy‑to‑use hemostat that stops hemorrhage in seconds could reduce preventable deaths in conflict and disaster zones and benefit civilian emergency medicine in low‑resource settings. On the other hand, militaries and humanitarian organisations will need to agree on protocols for training, supply chains, casualty triage and safe removal when definitive surgical care is available. If the powder becomes a standard of care for combat medics, it will also influence how combat casualty care is organised. The KAIST team’s explicit military collaboration highlights both the life‑saving intent and the practical design constraints of the project.
AGCL is a strong example of how materials science can compress a physical reaction (ionic gelation) and biological needs (hemostasis, infection control, tissue regeneration) into a compact product idea. The concept is elegant and the preclinical data are encouraging; for clinicians and military medics the question now is when and how the powder will pass through the rigorous safety and efficacy funnel that separates laboratory breakthroughs from everyday medical tools. Until human studies are completed and regulators sign off, AGCL will remain a promising — but not yet clinically available — step toward faster, more adaptable hemorrhage control.
Sources
- Advanced Functional Materials (research paper: "An Ionic Gelation Powder for Ultrafast Hemostasis and Accelerated Wound Healing").
- Korea Advanced Institute of Science and Technology (KAIST) — research repository and press materials on AGCL powder.